MedSource Labs Moves the Needle in the Peripheral Safety IV Market With the announcement of ClearSafe Comfort® and TrueSafe Comfort® Blood Control IV Catheters with groundbreaking active check valve technology.
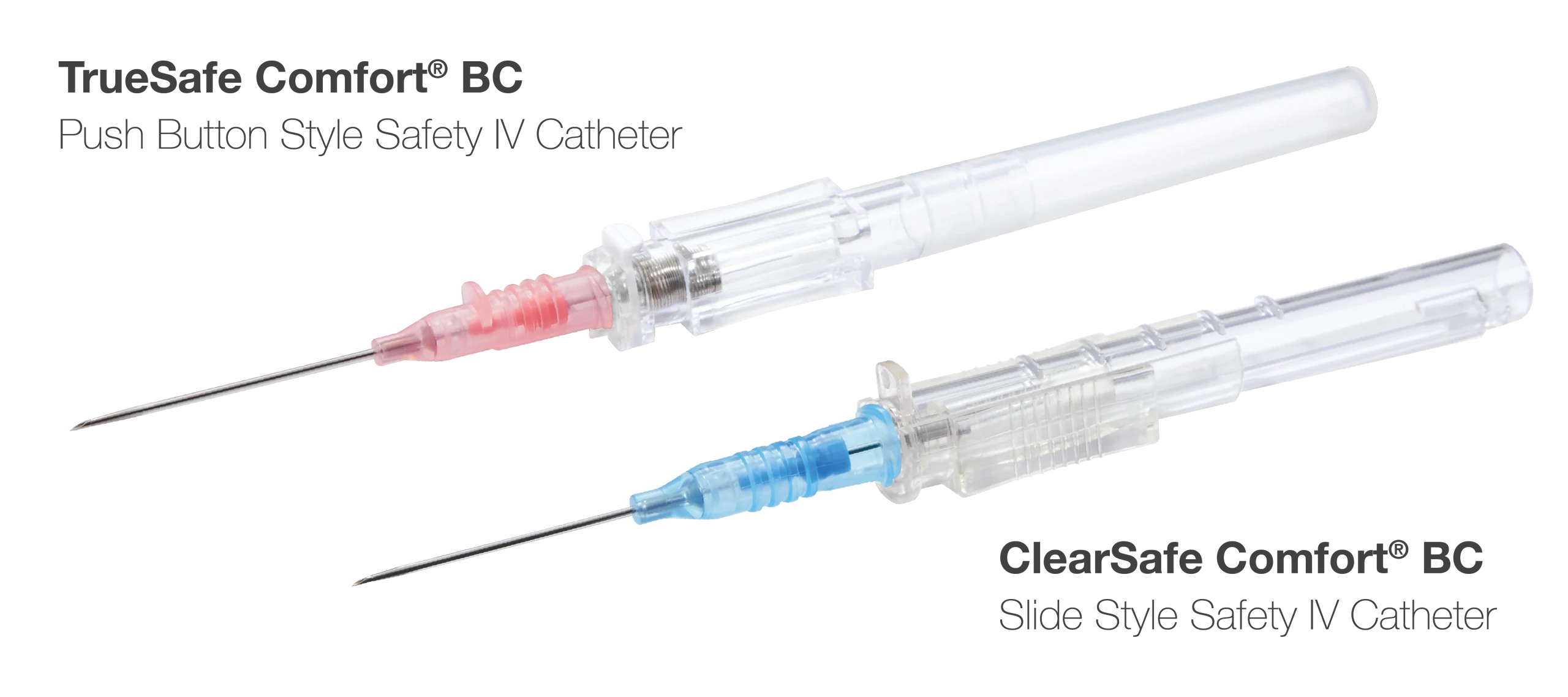
These devices conform to the U.S. Needlestick Safety and Prevention Act.
MedSource Labs is a leading medical product developer and supplier specializing in high-quality products and innovative medical product development.
The ClearSafe Comfort® and TrueSafe Comfort® Blood Control Safety IV Catheters are part of MedSource Labs’ high-performing IV catheter lines focused on caring for the caregiver. “Quality has always been a key component of our IV catheter products, and the blood control products raise the bar on medical staff safety and patient care,” said Kunelius.
MedSource Labs is ISO 13485:2016 certified for quality management in medical device manufacturing, registered with the FDA, and the blood control catheters are 510(k) Cleared Products.

About MedSource Labs
MedSource Labs is a leading provider of quality medical products, specializing in high-quality products and equipment and innovative medical product development. For nearly two decades MedSource Labs has been a trusted provider of quality medical products at a superb value. Recently, MedSource Labs has expanded into new markets and grown its services and offerings, including the Biomedix-WAI U.S. manufacturing arm and EMERGE product innovation process.
Contact
Jeremy Belloit | VP Marketing and Communications
jbelloit@medsourcelabs.com