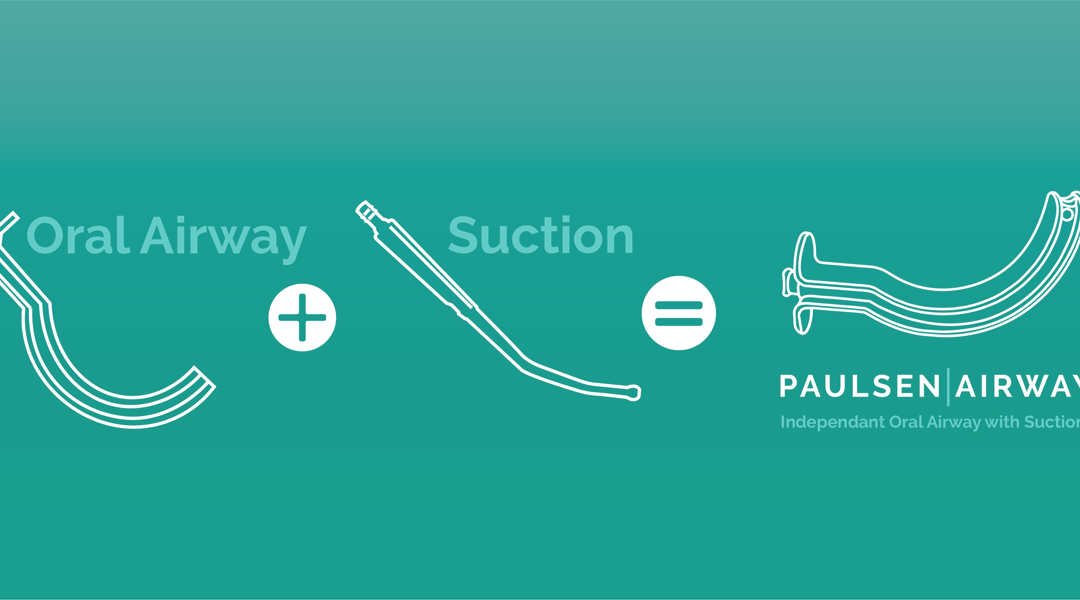
CHANHASSEN, Minn. – April 5, 2022—MedSource Labs introduces the Paulsen Airway™, an independent oral airway and suction catheter combined into one disposable medical device. The Paulsen Airway™ has many considerable benefits compared to traditional methods.
- Innovation: the Paulsen Airway™ is anatomically engineered to the patient’s airway allowing the customized suction catheter to sit in the hypopharynx at an optimal
- Safety: the multi-functionality of the device decreases the chance of cross contamination from devices being repeatedly introduced into the airway after being exposed to hospital 1
- Sustainability: the Paulsen Airway™ negates the need for a separate suction catheter, eliminating up to 200,000 pounds* of single-use plastics per 1,000,000
“Traditional suction catheters can be difficult to reach deep into patients’ airways, especially when oral airways are in use,”explained Heath Paulsen, CRNA, Veteran, and creator of the
Paulsen Airway. “When providing anesthesia on missions overseas, the need to rely on one product to maintain airway patency was clear,” he added. “Repeated attempts to clear the airway with the traditional system can lead to increased airway trauma, delayed suction time, and ineffective airway clearance. The Paulsen Airway™ only needs to be inserted once to control all the variables—no matter the environment.”
“Utilizing our EMERGE process, MedSource Labs takes a comprehensive approach to the development and manufacturing of quality medical devices such as the Paulsen Airway™,” said Rachel Sender, Vice President of Product Development at MedSource Labs. “Bringing the Paulsen Airway™ to market through the EMERGE process is evidence of the depth of our expertise and capacity to partner with inventors to bring a seemingly simple yet quite complex device through various phases of development, testing, and manufacturing.”
ABOUT MEDSOURCE LABS
Celebrating 20 years as a trusted provider of superior quality medical products, we specialize in customized manufacturing and innovative product development using our comprehensive EMERGE process—transforming product ideas into market-ready medical devices.
References:
- Brown, M., Williams, D. “Colonization of Yankauer Suction Catheters with Pathogenic Organisms.” American Journal ofInfection Control. Vol. 33, Issue 8. (October 2005): 483-485.
*Estimation based on MS-yk20 suction catheter
CONTACT:
Aimee Fahey | Communicationsafahey@medsourcelabs.com 952-241-8344